Peter T. Chivers, Priyanka Basak, and Michael J. Maroney have published a paper on the overlooked role of low molecular weight metal–ligand complexes, particularly L-histidine, in microbial nickel uptake, gene expression, and metalloenzyme maturation. This review explores the coordination chemistry of Ni(II)–histidine complexes and their fundamental importance to cellular metal homeostasis.
Highlights:
- Low molecular weight metal complexes play important roles in many physiological processes.
- L-His is a component of cytosolic metal buffers with which other biomolecules compete.
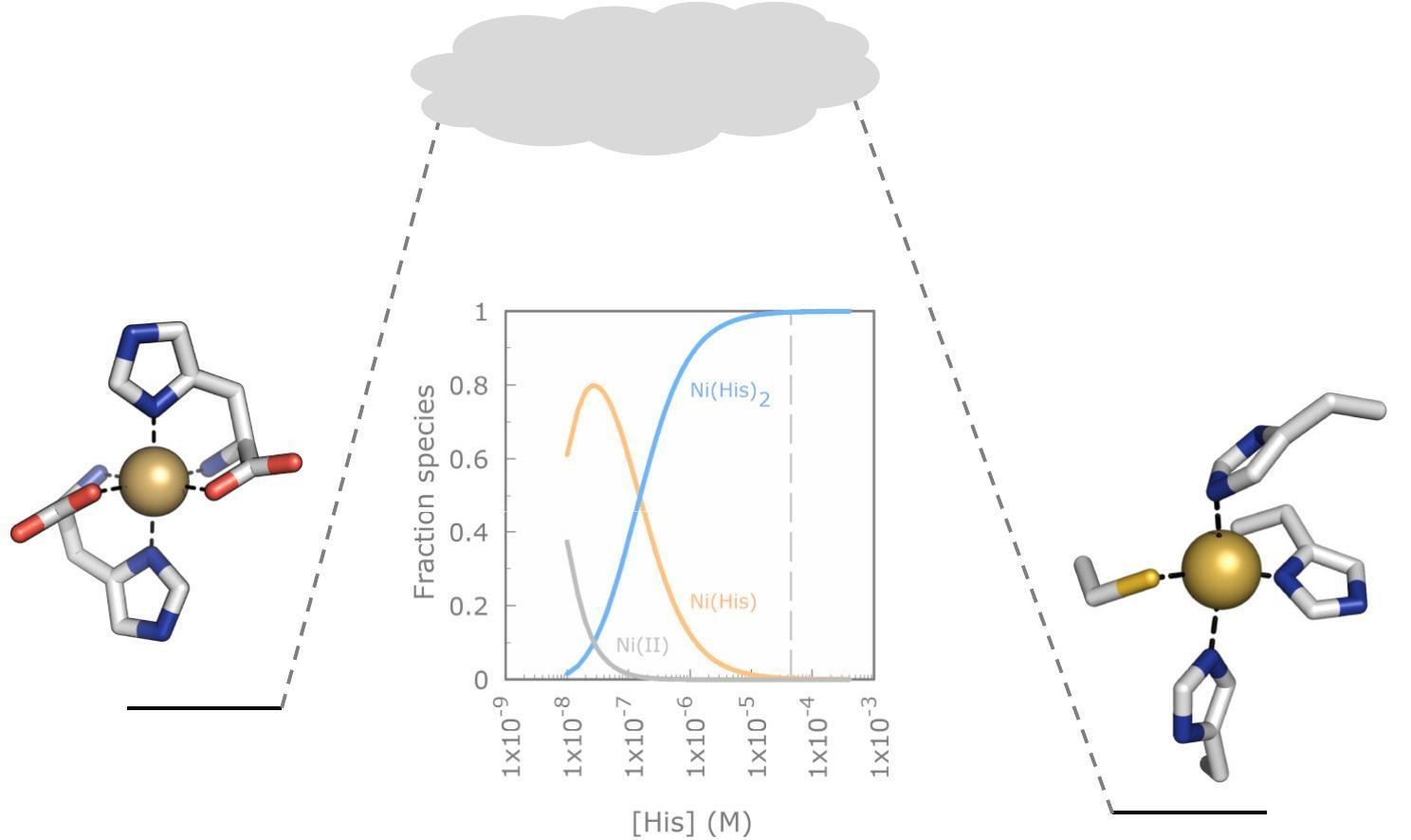
- At cellular Ni and L-His concentrations, Ni(His)+ and Ni(His)2 both play roles.
- Ternary complexes are important for buffering, homeostasis, and enzyme maturation.
https://doi.org/10.1016/j.jinorgbio.2024.112668
Abstract
Biological environments present a complex array of metal-binding ligands. Metal-binding proteins have been the overwhelming focus of study because of their important and well-defined biological roles. Consequently, the presence of functional low molecular weight (LMW) metal-ligand complexes has been overlooked in terms of their roles in metallobiochemistry, particularly within cells. Recent studies in microbial systems have illuminated the different roles of L-histidine in nickel uptake, gene expression, and metalloenzyme maturation. In this focused critical review, these roles are surveyed in the context of the coordination chemistry of Ni(II) ions and the amino acid histidine, and the physico-chemical properties of nickel complexes of histidine. These complexes are fundamentally important to cellular metal homeostasis and further work is needed to fully define their contributions.